SPOILER ALERT!
The Key of Function Of Caustic Soda In Soap Making That No-one is Speaking About
Wait for each to chill somewherebetween ninety five° and one hundred and five°.This is crucial for cleaning soap making.Too low and it’ll come collectively rapidly, but be coarse and crumbly. acoustic soda moves much more shortly with warm ingredients. It also is kind of useful to use the lye resolution whereas still heat. (When you combine the lye with the water, a chemical reaction caustic soda sds occurs that provides off warmth.) The residual warmth from the chemical response is sufficient to warm the oils and move things along. I actually have been informed many instances that it is extremely important to have the oils and the lye answer at certain temperatures to be able to efficiently make cleaning soap.
Thus, the actual fact they ingested caustic fragments doesn't remove the potential for stenosis, even of a severe grade. We imagine that the severity of esophageal lesions depends, along with the time of contact of the substance with the organism, on the amount ingested and on its concentration. It has been previously demonstrated13 that lesions provoked by caustic soda are probably caustic soda the most extreme, and are more severe than those provoked by alkaline brokers current in dishwashing merchandise. Stenosis occurred in 46.9% (23/49) of the sufferers who ingested "fragments" and in 93.6% of the sufferers who ingested a number of tablespoonfuls of caustic. With the ingestion of two or three tablespoonfuls, the risk of fistulas, perforations or even dying is elevated.
The base has already gone through the saponification course of, so you won't have to deal with lye. First, purchase pre-made blocks of uncolored, unscented cleaning what is caustic soda soap “base” from a craft store or soap supplier. The soap base is then melted in a microwave or a double boiler.
I’m lye for soap making in regards to the cleaning soap they have to use and what's in it. And yet, this dangerous chemical is among the primary elements of all soaps, each homemadeand retailer bought. Old timers will tell tales of how harsh lye cleaning caustic soda liquid soap is on the skin, however how properly it cleans clothes. Lye, or sodium hydroxide, is a very caustic chemical capable of causing serious harm. It can burn skin, cause blindness, and even cause dying if ingested.
When the cleaning soap is absolutely melted you possibly can add fragrance, shade, and additives. Pour the combination into a mould and the soap is able to use when it hardens. Soap is the results of a basic chemical reaction between fats or oils and lye. The strategy of attaining the chemical response sodium hydroxide suppliers is known as saponification. By fastidiously choosing a combination of quality oils, including your favorite fragrance or important oils, and swirling in a vigorous colorant, your handmade cleaning soap all of a sudden takes on a captivating, rustic character.
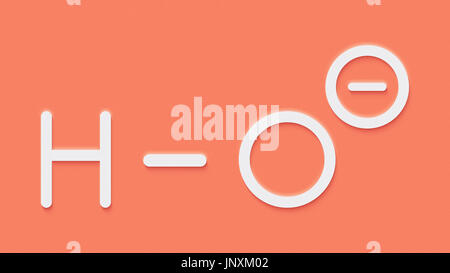
If the product is at a pH above 7, then it'll have extra hydroxide ions than water. In this case, have a look at the pH – the higher the pH, the more harmful the hydroxide shall be. In common, anything beneath pH 10 is okay if it’s involved with your pores and skin for a short sodium hydroxide suppliers amount of time, although you’ll most likely need to rinse your pores and skin afterwards (cleaning soap is pH 9-10). Sodium hydroxide, with the method NaOH, is often used in merchandise as a pH adjuster. It’s additionally utilized in soap-making to show fats and oils into cleaning soap.
Depending on the aim, you might be able to substitute a chemically similar sturdy base, potassium hydroxide (KOH). This is a chemical you possibly can, in case you are extraordinarily devoted, make yourself by soaking wooden ashes in water. To do that, soak a large amount of ashes in a small quantity of water.
If we add NaOH to water, it dissociates into Na+ and OH-. The sodiums do not do something necessary, however the hydroxyls make the solution caustic soda manufacturers extra basic. Making soap with a soften and pour base is safe, straightforward, and convenient.
Thus, the actual fact they ingested caustic fragments doesn't remove the potential for stenosis, even of a severe grade. We imagine that the severity of esophageal lesions depends, along with the time of contact of the substance with the organism, on the amount ingested and on its concentration. It has been previously demonstrated13 that lesions provoked by caustic soda are probably caustic soda the most extreme, and are more severe than those provoked by alkaline brokers current in dishwashing merchandise. Stenosis occurred in 46.9% (23/49) of the sufferers who ingested "fragments" and in 93.6% of the sufferers who ingested a number of tablespoonfuls of caustic. With the ingestion of two or three tablespoonfuls, the risk of fistulas, perforations or even dying is elevated.
Why is caustic soda used in soap?
Caustic soda causes saponification and is an essential ingredient in soap-making. When flakes or beads of sodium hydroxide get added to a liquid, it forms a lye solution. This solution, when mixed with oils or fats, will lead to the chemical reaction called saponification.
Related Questions
The base has already gone through the saponification course of, so you won't have to deal with lye. First, purchase pre-made blocks of uncolored, unscented cleaning what is caustic soda soap “base” from a craft store or soap supplier. The soap base is then melted in a microwave or a double boiler.
- In agreement with this idea, our knowledge show that even though 93.7% of the kids ingested small portions of caustic agent, i.e. "fragments", 50% of them introduced stenosis.
- When the stenosis was categorised by radiology, the youngsters were found to point out a 10% fee of severe stenosis (Table 4).
I’m lye for soap making in regards to the cleaning soap they have to use and what's in it. And yet, this dangerous chemical is among the primary elements of all soaps, each homemadeand retailer bought. Old timers will tell tales of how harsh lye cleaning caustic soda liquid soap is on the skin, however how properly it cleans clothes. Lye, or sodium hydroxide, is a very caustic chemical capable of causing serious harm. It can burn skin, cause blindness, and even cause dying if ingested.
When the cleaning soap is absolutely melted you possibly can add fragrance, shade, and additives. Pour the combination into a mould and the soap is able to use when it hardens. Soap is the results of a basic chemical reaction between fats or oils and lye. The strategy of attaining the chemical response sodium hydroxide suppliers is known as saponification. By fastidiously choosing a combination of quality oils, including your favorite fragrance or important oils, and swirling in a vigorous colorant, your handmade cleaning soap all of a sudden takes on a captivating, rustic character.

If the product is at a pH above 7, then it'll have extra hydroxide ions than water. In this case, have a look at the pH – the higher the pH, the more harmful the hydroxide shall be. In common, anything beneath pH 10 is okay if it’s involved with your pores and skin for a short sodium hydroxide suppliers amount of time, although you’ll most likely need to rinse your pores and skin afterwards (cleaning soap is pH 9-10). Sodium hydroxide, with the method NaOH, is often used in merchandise as a pH adjuster. It’s additionally utilized in soap-making to show fats and oils into cleaning soap.
Depending on the aim, you might be able to substitute a chemically similar sturdy base, potassium hydroxide (KOH). This is a chemical you possibly can, in case you are extraordinarily devoted, make yourself by soaking wooden ashes in water. To do that, soak a large amount of ashes in a small quantity of water.
If we add NaOH to water, it dissociates into Na+ and OH-. The sodiums do not do something necessary, however the hydroxyls make the solution caustic soda manufacturers extra basic. Making soap with a soften and pour base is safe, straightforward, and convenient.
